
Liquid Chloroform at Rs 55/kg Pune ID 2851062413262
Thin Layer Chromatography (TLC) is an extremely useful technique for monitoring reactions. It is also used to determine the proper solvent system for performing separations using column chromatography. TLC uses a stationary phase, usually alumina or silica, that is highly polar (standard) or non-polar (reverse phase), and a mobile phase, some.

Chloroform, 1 liter Lösningsmedel Tillbehör HPLC Kromatografitillbehör Produkter
Chloroform, or trichloromethane (often abbreviated as TCM ), is an organic compound with the formula C H Cl 3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and in turn PTFE. [10]

Chloroform Nkazi Sciences
For example, carbon tetrachloride, CCl 4, is nonpolar, but chloroform, CHCl 3, and methyl chloride, CH 3 Cl are polar:. The table below shows whether the examples in the previous sections are polar or nonpolar. For species which have an overall charge, the term "charged" is used instead, since the terms "polar" and "nonpolar" do.
The theoretical values of the IPCE and ΔGinj (a) in polar solvents,... Download Scientific Diagram
Chloroform | CHCl3 | CID 6212 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.. It has a role as an inhalation anaesthetic, a non-polar solvent, a carcinogenic agent, a central nervous system drug and a.

Best Overview Is CHCl3 Polar or Nonpolar Science Education and Tutorials
Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct question is not "is it polar or non-polar" but "how polar is it." Nonetheless, guidelines have been created to make it easier.

chloroform Jennifer Hallock
CHCl3 is an organic compound known by its IUPAC name as trichloromethane. It is also commonly known by the term chloroform. It exists as a colorless dense liquid having a sweet smell. Many of you might have doubts regarding whether CHCl3 is polar or not.

Phytochemical constituents identified in the nonpolar (chloroform)... Download Table
Chloroform the molecule is polar, as you said. Chloroform the solvent is "nonpolar" because it has a low dielectric constant. Check in wiki here - you can see that chloroform has a dipole moment that is not super high, but nonzero, however its dielectric constant is much lower than those solvents classified as "polar". 3.

Chloroform Stock Footage & Videos 13 Stock Videos
Why? The dielectric constant of chloroform is 4.81 F/m which, being a small value makes it nonpolar. What are Polar Solvents? Polar solvents are the solvents that have a polar bond, the basis of which is the electronegativity difference between constituent atoms.

Nonpolar and polar lipid structures which represent lipids found... Download Scientific Diagram
Lipids are all insoluble in polar solvents like water but highly soluble in the non-polar or weakly polar organic solvents, including ether, chloroform, benzene, and acetone. In fact, these four solvents are often referred to as "lipid-solvents" or "fat-solvents". Other biomolecules such as amino acids, proteins, carbohydrates, and nucleic.

Chloroform, Packaging Type HDPE Barrel, 220 kg at Rs 45/kg in Coimbatore
Learn to determine if CHCl3 (Trichloromethane or Chloroform) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape)..more.more Polar and Nonpolar.

Carotenoid extracted from A, B, C and D using MgSilicate, CaSilicate,... Download Scientific
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education and Tutorials
About Transcript Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge.
Chloroform
Expand/collapse global location. 6.05.1. Protic vs Aprotic Solvents. Page ID. Solvents used in organic chemistry are characterized by their physical characteristics. Among the most important are whether the solvents are polar or non-polar, and whether they are protic or aprotic. Because non-polar solvents tend to be aprotic,the focus is upon.

Chloroform's Gallery Pixilart
The polarity of a Molecule. The molecules fall into the following categories concerning molecular polarity. The molecule is nonpolar if there is no polar bond in it, e.g., H-H, F-F, and CH 4 are nonpolar molecules. Fig. 3.8.4 illustrates CH 4 molecules with green color electron clouds that represent a nonpolar molecule.. Figure \(\PageIndex{4}\): Methane (CH 4) with no polar bond is nonpolar.

Chloroform Lab preparation, Properties, Uses and Question/Answer
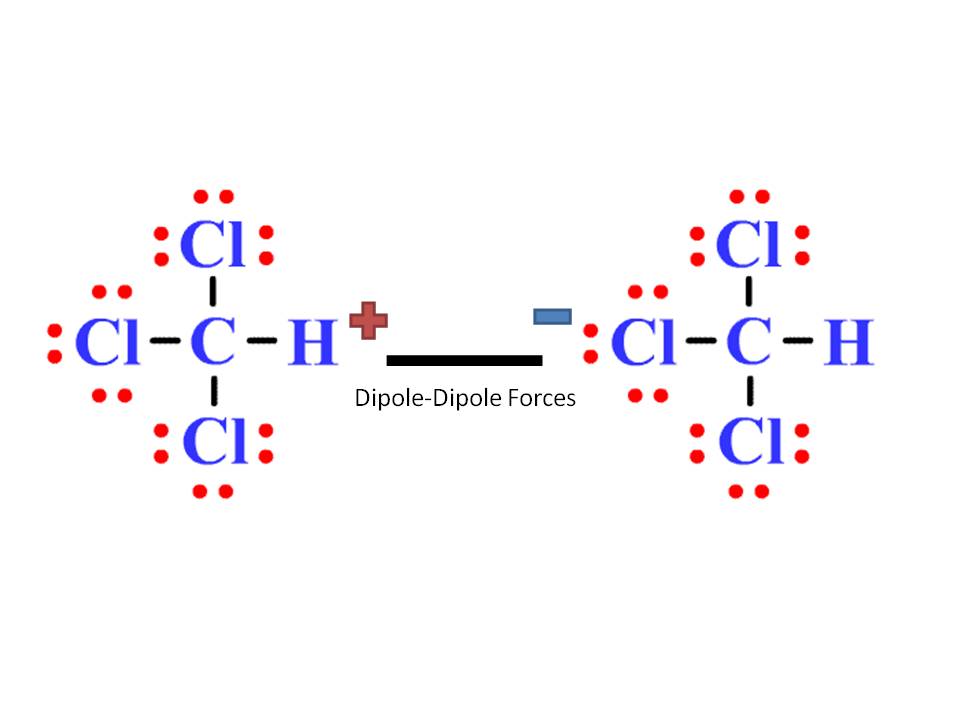
Chloroform (CHCl3) is a polar molecule. Chlorine (Cl) is a highly electronegative element. It attracts the shared electron cloud of each of the three C-Cl bonds as well as the C-H bond. Oppositely charged poles develop in the molecule. CHCl3 has an apparently symmetric tetrahedral shape or geometry.
The Chemistry of Chloroform
Polarity of Solvents. Water Acetic Acid Ethyleneglycol Methanol Ethanol Isopropanol Pyridine Acetonitrile Nitromethane Diehylamine Aniline Dimethylsulfoxide Ethylacetate Dioxane Acetone Dicholoroethane Tetrahydrofuran Dicholoromethane Chloroform Diethylether Benzene Toluene Xylene Carbontetrachloride Cyclohexane Petroleum ether Hexane Pentane.